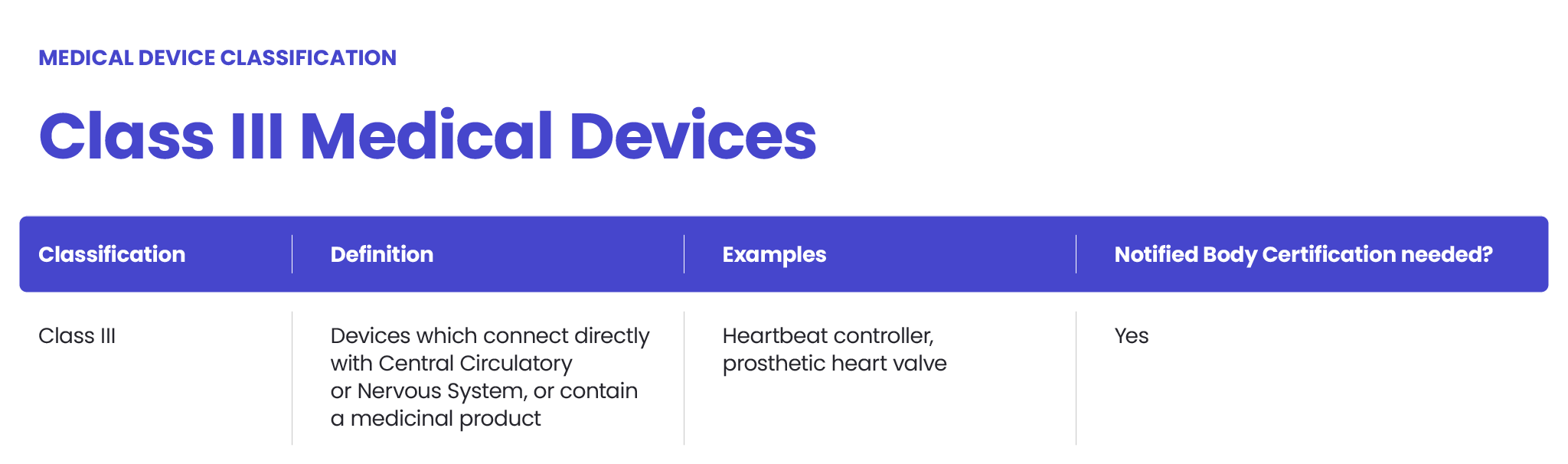
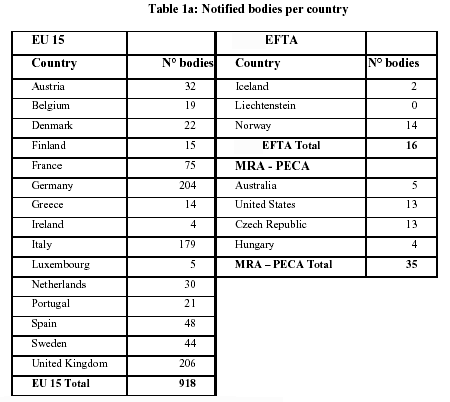
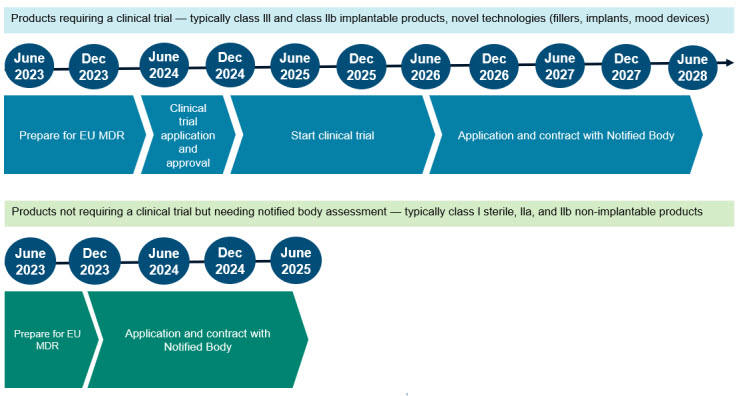
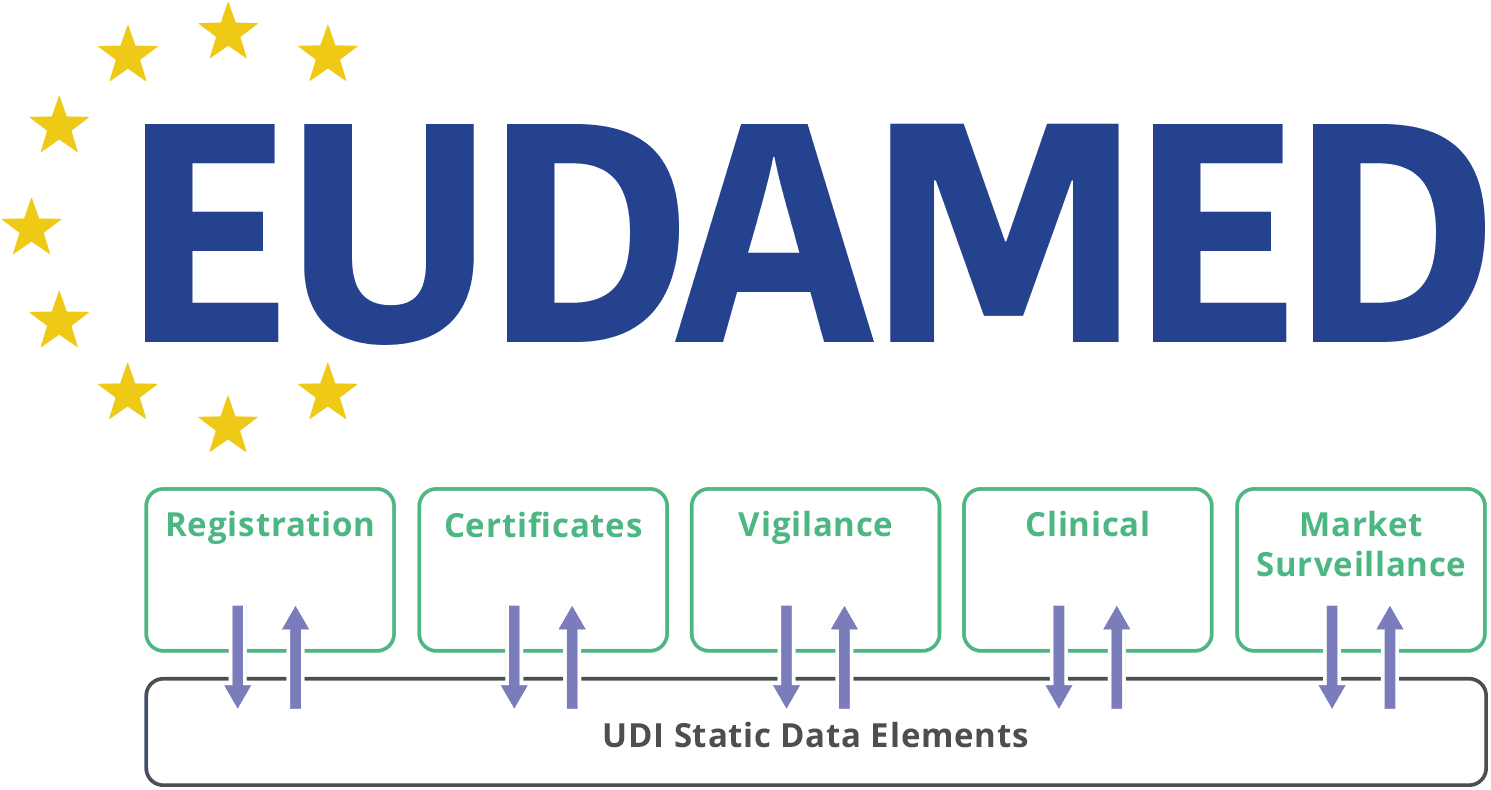
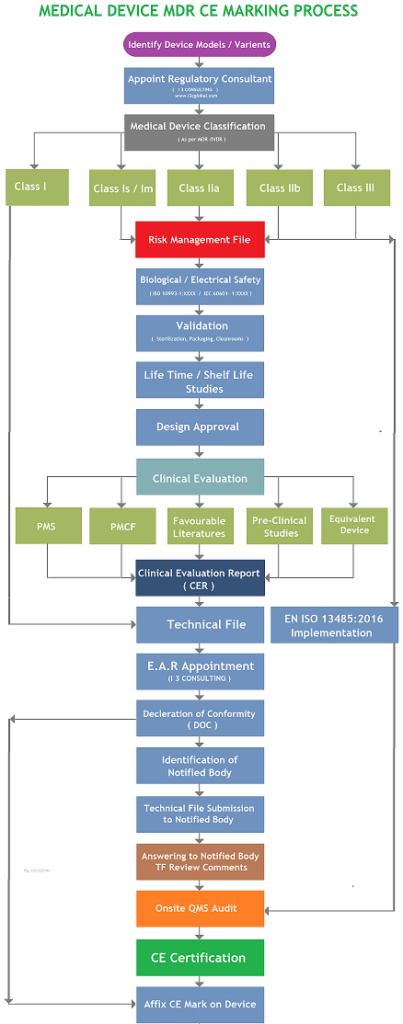
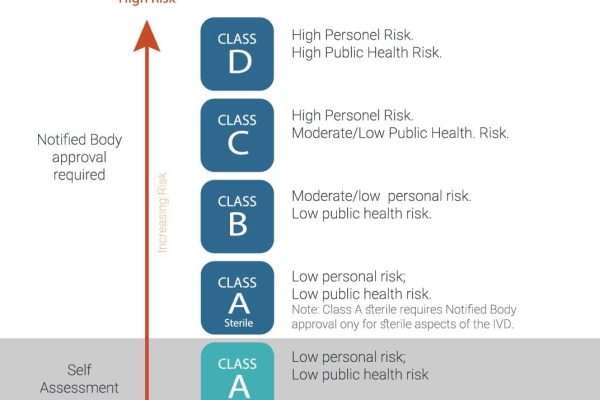
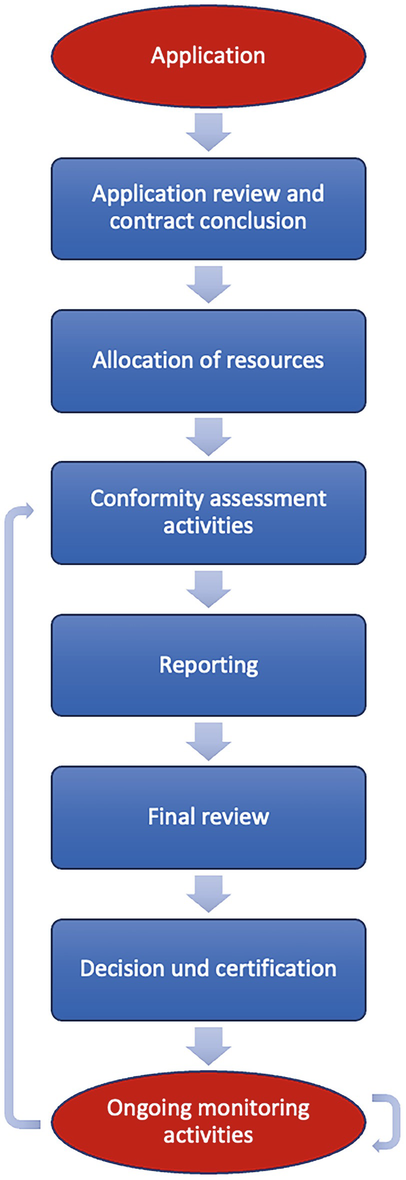
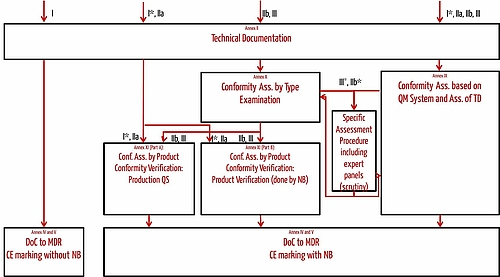
Application for a Notified Body Opinion according to Article 117, Regulation (EU) 2017/745 on Medical Devices

Medical device legislation for custom-made devices after the UK has left the EU: answers to ten important questions | British Dental Journal